Pathogen Environmental Monitoring Program (EMP) for Food Safety
An effective environmental monitoring program (EMP) is a pillar of food safety for validating and verifying the effectiveness of preventive controls within a processing facility. The primary components of an EMP include:??
Why Do Environmental Monitoring?
In most circumstances, an environmental monitoring program is a requirement established by regulation to ensure manufacturers are controlling environmental pathogens and allergens.?Beyond that, customer expectations and food safety auditing schemes generally require environmental monitoring. Environmental monitoring also can be an effective tool to help resolve product spoilage and shelf-life issues.?
Key Considerations for Implementing an Environmental Monitoring Program?
When is Environmental Monitoring Required?
Ready-to-eat (RTE) foods or ingredients exposed to the environment before packaging and that do not receive a control measure to minimize pathogens, will require a sanitation verification activity. All food?stock-keeping units (SKUs)?that require clean food contact surfaces for allergen removal will require cleaning verification activity.?
WHAT ARE THE GOALS OF AN ENVIRONMENTAL MONITORING PROGRAM??
Primary?
to find pathogens or allergens in the environment before they contaminate the product.?
Secondary?
to find spoilage microorganisms in the environment before they affect the product.?
Tertiary?
to assess the effectiveness of cleaning, sanitation, and employee hygiene practices.
WHAT ARE YOU TRYING TO CONTROL IN AN EMP?
Salmonella is the target microorganism for environmental monitoring of product-contact and non-product-contact surfaces in a low-moisture food manufacturing facility.?
Listeria monocytogenes is the target microorganism for environmental monitoring of product-contact and non-product-contact surfaces in a high-moisture food manufacturing facility.?
Allergens that require food contact surface cleaning between SKUs.?
How Do You Achieve Salmonella,?L. monocytogenes, and Allergen?Control??
WHERE TO TEST IN THE ENVIRONMENT??
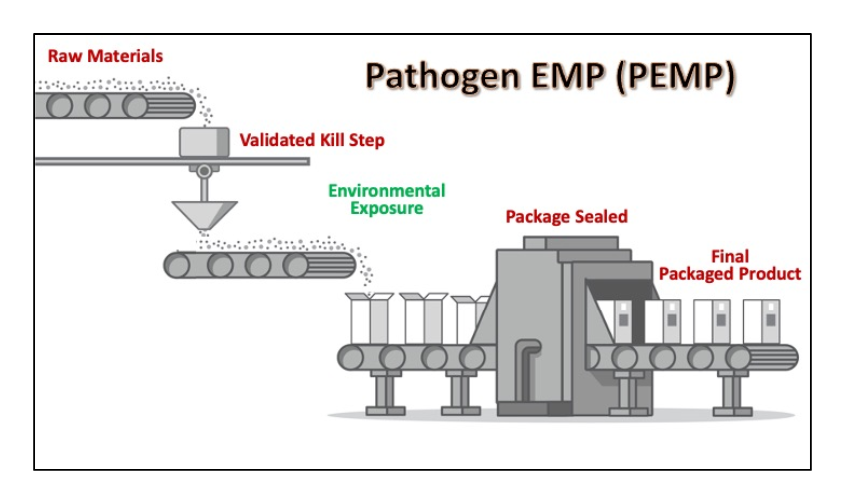
A well-designed environmental monitoring program will include samples from various areas throughout your production process. For pathogen control, the focus of an environmental monitoring program should be on the Primary Microbial Control Area. This area is defined as the area subsequent to the lethality step up to the packaging step. For processes that do not have a Salmonella or Listeria lethality step, the entire processing area is considered the Primary Microbial Control Area.?For allergen control, the primary focus is to ensure the cleanliness of food contact surfaces.?
The Zone Concept?
The simplest way to organize your sampling program is through the use of zones as outlined below. Multiple sampling sites from each zone should be determined (based on your specific facility design and processes) before you begin taking samples. You can rotate sites at each sampling interval to increase coverage in a particular zone. For example, if you have identified 60 potential sampling sites in Zone 2, randomly sample a few of these sites each week, making sure that each site is sampled at least once per month. This system will help you stretch your testing budget while making sure you sample all sites needed to verify program effectiveness.?Larger, high-risk facilities will require a greater number of samples and perhaps a greater sampling frequency than smaller, low-risk facilities.?
Identification of Sampling Sites?
Establishment of monitoring locations should be based on previous studies during dynamic conditions.?This could include a “gridding” or “mapping” study to determine worst case/most meaningful locations, as well as considerations of all hot spots or high-risk areas.?
Keep a detailed sampling site log and facility map that details locations of sampling sites. The log should contain written detail on how to collect samples from difficult-to-access areas (sites under processing equipment, sites within equipment, overhead fixtures, etc.). Note that the number of sampling sites and number of samples will vary with the type of product, nature of processing scheme, and risk level deemed acceptable.?
Higher-risk products processed in a large complex facility will likely need more samples and a greater sampling frequency than lower-risk products processed in a smaller, more simple processing facility.?
Sampling Frequency?
When first starting, sample numerous sites and use large surface areas to gauge the degree of contamination and identify potential problem areas.?
Increase frequency following adverse events or process changes, such as:?
Testing Frequency?
Testing frequency should be based on:?
ZONE MANAGEMENT?
Zone 1: Direct Product Contact Surfaces?
Surfaces where food product is exposed to the environment before final package closure.?
Locations?
Tables, conveyor belts, buckets, fillers, hoppers, utensils, employee hands and gloves, items and surfaces directly over or in close proximity to direct food contact surfaces such as lights fixtures and piping, compressed air lines, and water filters.?
Tests?
Pathogen testing of Zone 1 surfaces is a controversial subject due to potential for recalls if found (Salmonella and/or L. monocytogenes). As a result, many decide to test for?appropriate indicator bacteria (Listeria species, aerobic plate count, coliform count, or total Enterobacteriaceae count).?Testing for allergens requiring removal is appropriate.?
Frequency of Testing?
Daily or weekly depending on risk.?
Minimum Number of Samples?
Depends on facility size, process complexity, and level of risk.?
Program Validation?
Necessary if only using indicator (microbial indicators, ATP) testing in the routine program. Validation includes periodic testing for pathogens and allergens of concern and the indicator to develop a correlation between the level of the chosen indicator and the prevalence of the pathogen or allergen.?
Product Disposition?
All product produced on the line tested should be held until final results are received when testing any Zone 1 sites for pathogens or allergens.?
Zone 2: Non-Product Contact Surfaces Close to Zone 1 Surfaces?
Locations?
Equipment frames, drip shields and pans, control panels and buttons, overhead fixtures and piping not directly over or in close proximity to food contact surfaces, computer screens, maintenance tools.?
Tests?
Salmonella and/or L. monocytogenes,?indicator bacteria (Listeria species, aerobic plate count, coliform count, total Enterobacteriaceae count).?Allergen testing is typically not done on Zone 2, 3, or 4 surfaces unless risk assessment justifies.?
Frequency of Testing?
Weekly.?
Minimum Number of Samples?
Depends on facility size, process complexity, and level of risk.?
Zone 3: Non-Product Contact Surfaces in Open Processing Area?
Locations?
Floors, walls, ceilings, drains, hoses, cleaning equipment including brooms and brushes, air handling units, condensate drip pans, carts, pallets, forklifts, trash cans, foot baths, sink area including soap and towel dispensers.?
Tests?
Salmonella and/or L. monocytogenes, indicator bacteria (Listeria species, aerobic plate count, coliform count, total Enterobacteriaceae count, lactic acid bacteria count).?Allergen testing is typically not done on Zone 2, 3, or 4 surfaces unless risk assessment justifies.?
Frequency of Testing?
Weekly.?
Minimum Number of Samples?
Depends on facility size, process complexity, and level of risk.?
领英推荐
Zone 4: Support Facilities Not in Open Processing Area?
Locations?
Bathrooms, locker rooms, cafeteria and break rooms, office rooms, hallways, warehouse, loading docks, maintenance shop, storage areas.?
Tests?
Salmonella and/or L. monocytogenes, indicator bacteria (Listeria species, aerobic plate count, coliform count, total Enterobacteriaceae count, lactic acid bacteria count).?Allergen testing is typically not done on Zone 2, 3, or 4 surfaces unless risk assessment justifies.?
Frequency of Testing?
Monthly to quarterly.?
Minimum Number of Samples?
Depends on facility size, process complexity, and level of risk.
ASEPTIC COLLECTION OF ENVIRONMENT SAMPLES
Collecting environment samples in an aseptic manner is critical to ensuring the quality of the testing results. If the person collecting the samples contaminates the specimen, the laboratory result will not accurately reflect the condition of your manufacturing environment.
Key points for sample collection include:?
Sampling Tools?
There are multiple commercial sampling tools available for use. This instruction addresses three of the most common types.?Most tools are pre-sterilized with a transport buffer.?It is important to match the correct sampling tool for the target analyte – micro tools for collecting microorganisms and allergen tools for collecting allergen residue.?
Sponge in Bag?
A small sterile sponge (about 1” x 2”) is pre-moistened with a transport buffer in a sealed bag. Some vendors attach a pair of sterile gloves to the sponge bag.?
Sponge with Handle (“Spongesickle” or “Stick-Sponge”)?
A small sterile sponge is attached to the end of a long plastic handle; the whole unit is contained in a sealed plastic bag containing a small amount of transport buffer.?
Swab (“Q-tip” style)?
A small sterile pre-moistened swab resembling a Q-Tip is contained in a tube with transport buffer.?
Sponge/Swab Transport Buffers?
The most common transport buffers contain various agents that act to neutralize common sanitizers that might be present in the collected samples. Any residual sanitizer could have bactericidal and/or bacteriostatic activity, which could negatively impact the ability to count or recover the target microorganisms by enrichment and/or by direct impact on downstream detection methods. The three most common neutralizing buffers are Letheen broth, Neutralizing buffer, and D/E broth. Their neutralizing components and sanitizers inactivated are listed below.?
Letheen Broth?
Neutralizing Buffer?
D/E Broth?
Due to differences in the composition of these three common transport buffers not all are compatible with all detection methodologies. For example, D/E broth is purple and can affect detection methods that require visual observation of a color change, such as Petrifilm or others. Additionally, the neutralizing agents themselves (e.g., aryl sulfonate complex) can have negative effects on some detection technologies.??
It is important to select the transport buffer that gives the broadest protection against the sanitizers most commonly used in the production process. Potential impacts on the selected detection method may need to be considered particularly if a change in transport buffer is implemented. Several buffers such as HiCap? neutralizing buffer have been developed and optimized for use with both cultural and PCR based detection methods.?
?EMP DATA INTERPRETATION & MANAGEMENT?
Types of Data?
What Do the Numbers Mean??
Note: not detected, negative, or absent in a sample does not mean entire area is free of contamination.?
Record Keeping?
Establishment of Performance Targets?
Collect preliminary indicator counts for each location and determine a population level for each indicator to serve as a target level. For each indicator, also establish a population level that is unacceptable.?
Example Performance Targets?
Establishment of an Action Plan?
If pathogen or allergen are found within Zone 1, the product that was produced along that surface may be considered adulterated. Therefore, timely action must be taken for the product and the process.?
The Action Plan should consider:??
Forming a Response Team?
An OOS response team should include individuals knowledgeable and representative of each phase of the process. The team may include:?
What to Do When Elevated Indicator Counts are Found??
Corrective Actions if Salmonella/Listeria monocytogenes is Found?
??Routine Preventative Controls
References
1- Food and Drug Administration ( FDA )
2- Food and Agriculture Organization ( 联合国粮农组织 )
4- 美国加州大学戴维斯分校
5- 美国普渡大学
7- 3M 公司
Thank you for your time!
F&B Industry Technical Sales | Sanitation & Disinfection | Calvatis Vietnam
5 个月I'd love to see this information from you. Wishing you all the best ! From Calvatis Vietnam